October 2022
August Longino, MD1, Tiffany Gardner, MD2, Brandon Fainstad, MD3
Section Editor: Josephine Cool, MD4
1 Resident, Internal Medicine, University of Colorado, 2 Chief Resident, Internal Medicine, University of Colorado, 3 Assistant Professor, General Internal Medicine, University of Colorado, 3 Instructor of Medicine, Harvard Medical School, Section of Hospital Medicine, Beth Israel Deaconess Medical School
Objective(s)
- Identify systolic dysfunction in the PLAX view and use end-point septal separation (EPSS) to characterize the severity.
- List the common valvular conditions and measuring errors that lead to an overestimated EPSS.
- Identify characteristics, including the “D-sign”, of increased right ventricular pressure in the parasternal short axis (PSAX) view.
- Generate a differential diagnosis for RV strain.
- Determine the appropriate next steps in management when a massive PE is suspected based on clinical presentation and focused cardiac assessment.
- Identify a pericardial effusion on bedside ultrasound in the parasternal long (PLAX) and distinguish it from a pleural effusion.
- Develop a brief differential diagnosis for pericardial effusion based on the clinical setting.
- Describe the key clinical features that suggest tamponade physiology, including how to perform pulsus paradoxus.
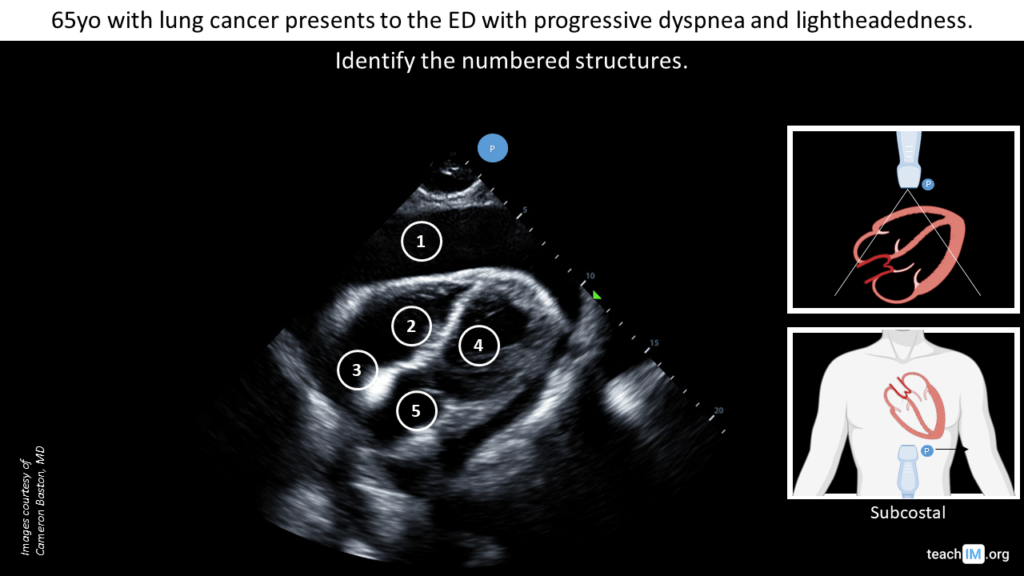
- Identify the cardiac chambers and pericardial space in a subcostal view.
- Identify RV collapse during diastole.
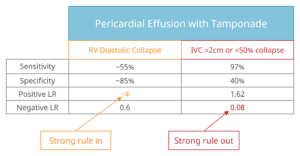
- Describe the test characteristics of right ventricular diastolic collapse and a plethoric IVC for the diagnosis of pericardial tamponade.
Teaching Instructions
Preparation: Plan to spend 45 minutes to an hour familiarizing yourself with the background information below, key findings on the ultrasound clips and the progressions of animations on the PowerPoint. This conference is intended as an introduction to clinical application of cardiac US and the second in our POCUS case conference series.
How to present: Present the PowerPoint by downloading the PowerPoint file (the US clips do not function in PowerPoint browser windows). Have the image pulled up in presenter mode before learners look at the screen to avoid revealing the diagnosis. Each case progresses through three or more questions, beginning with an overall interpretation, identification of a generalizable rule, and a clinical integration question. We recommend a pair-share structure with a junior and senior trainee. For each successive question, the presenter can elect to have pairs discuss their thoughts then ask for a volunteer to share or, to expedite the conference, simply ask for an audience response. Ask a learner to provide an overall interpretation. Advance using the keyboard arrows or mouse click to reveal subsequent questions and then answers with their accompanying graphics. You can go back to prior graphics and questions by using the back arrow or scrolling back on the mouse wheel.
Case 1: Severely reduced LVEF using EPSS (PLAX)
POCUS image/clip interpretation:. Global hypokinesis of LV with severely reduced LVEF and enlarged left atrium.
Clinical diagnosis: Acutely decompensated heart failure with reduced ejection fraction (HFrEF)
Teaching:
- Gross assessment of LV systolic function includes assessment of wall thickening and reduction in chamber size. An enlarged left atrium (larger than the LVOT and RV, ‘rule of thirds’) suggests increased left atrial pressures and may corroborate findings of reduced LV EF. However, an enlarged LA may also be due to mitral valve disease or LV diastolic dysfunction.
- E-point septal separation (EPSS) is an additional method to more objectively evaluate LV systolic function. The minimum distance between the anterior mitral valve leaflet and the septum is measured using M-mode.
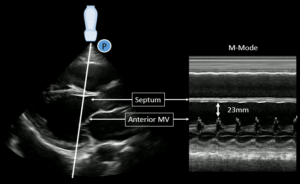
Increasingly large EPSS measurements correlate with worse systolic function:
- EPSS <7mm suggests a normal systolic function.
- EPSS >13mm suggests mod-severely reduced EF
Pitfall: EPSS may be over-estimated due to mitral stenosis, aortic regurgitation or a misaligned M-mode.
Case 2: RV strain from pulmonary embolus (PSAX)
POCUS image/clip interpretation: Parasternal short axis (PSAX) with dilated RV with flattened intraventricular septum (“D-sign) suggestive of RV strain.
Clinical diagnosis: RV strain in the setting of acute chest symptoms and shock, concerning for a massive pulmonary embolus.
Teaching:
- PSAX is the preferred view to identify RV strain with a dilated RV and flattened interventricular septum or “D-sign”.
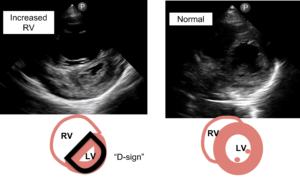
Pitfall: An off-axis PSAX may give the false appearance of a flattened septum. Always confirm the finding at multiple levels and views.
- The differential for increased RV pressure relative to the LV includes:
- Acute pulmonary embolus
- Chronic pulmonary hypertension
- RV systolic dysfunction
- Pulmonic valve disease
- The differential for increased RV pressure relative to the LV includes:
Case 3: Small pericardial effusion (PLAX)
POCUS image/clip interpretation – Parasternal long axis with small circumferential simple pericardial effusion. Normal left ventricular chamber size and function. Normal left atrial chamber size.
Clinical diagnosis – Suspected viral pericardial effusion without hemodynamic significance and no evidence of LV dysfunction to explain the patient’s presentation.
Teaching:
- In the PLAX view, a pericardial effusion is anterior to the aorta while a pleural effusion is posterior to the aorta.
- Most pericardial effusions do not cause tamponade physiology.
- The common clinical features of tamponade are JVD, tachycardia, and pulsus paradoxus >10.
Case 4: Malignant pericardial effusion with tamponade physiology (subcostal)
POCUS image/clip interpretation:. Large circumferential pericardial effusion with diastolic collapse of the right ventricle. Normal LV systolic function.
Clinical diagnosis: Malignant pericardial effusion with clinical tamponade.
Teaching:
- Subcostal cardiac view is the most sensitive view for identifying pericardial effusion.
- Ventricular diastole occurs when the tricuspid and mitral valves are open.
- Diastolic collapse of the RV has good rule in value (+LR 4) and the absence of a plethoric IVC (>2cm and <50% collapsing) has good rule out value (-LR 0.08) for the diagnosis of tamponade.
Presentation Board
Take Home Point
- Left atrial enlargement – The LA is much larger than the LVOT and RV (rule of thirds).
- Reduced systolic function – The cross-sectional area of the LV chamber does not decrease much in size and the walls do not thicken.
- E-point septal separation (EPSS) is measured with M-mode along the distal portion of the anterior mitral valve.
- Increasingly large EPSS measurements correlate with worse systolic function:
- An EPSS <7mm suggests a normal systolic function.
- An EPSS >13mm suggests mod-severely reduced EF
- Mitral stenosis, aortic regurgitation, or a misaligned M-mode may over-estimate EPSS.
- The PSAX is the preferred view for identifying RV strain with a dilated RV and flattened interventricular septum or “D-sign”.
- The differential for increased RV pressure relative to the LV includes:
- Acute pulmonary embolus
- Chronic pulmonary hypertension
- RV systolic dysfunction
- Pulmonic valve disease
- In the PLAX view, a pericardial effusion is anterior to the aorta while a pleural effusion is posterior to the aorta.
- Most pericardial effusions do not cause tamponade physiology. The common clinical features of tamponade are JVD, tachycardia, and pulsus paradoxus >10.
- Subcostal cardiac view is the most sensitive view for identifying pericardial effusion.
- Ventricular diastole occurs when the tricuspid and mitral valves are open.
- Diastolic collapse of the RV has good positive predictive value and the absence of RV and RA diastolic collapse has good negative predictive value for tamponade.
References
- Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. doi:10.1002/jhm.2434
- McKaigney CJ, Krantz MJ, La Rocque CL, Hurst ND, Buchanan MS, Kendall JL. E-point septal separation: a bedside tool for emergency physician assessment of left ventricular ejection fraction. Am J Emerg Med. 2014 Jun;32(6):493-7. doi: 10.1016/j.ajem.2014.01.045. Epub 2014 Feb 3. PMID: 24630604.
- Alerhand S, Sundaram T, Gottlieb M. What are the echocardiographic findings of acute right ventricular strain that suggest pulmonary embolism? Anaesth Crit Care Pain Med. 2021 Apr;40(2):100852. doi: 10.1016/j.accpm.2021.100852. Epub 2021 Mar 26. PMID: 33781986.
- Ruxandra Jurcut, Sorin Giusca, André La Gerche, Simona Vasile, Carmen Ginghina, Jens-Uwe Voigt, The echocardiographic assessment of the right ventricle: what to do in 2010?, European Journal of Echocardiography, Volume 11, Issue 2, March 2010, Pages 81–96, https://doi.org/10.1093/ejechocard/jep234
- Alerhand, S., & Carter, J. M. (2019). What echocardiographic findings suggest a pericardial effusion is causing tamponade? The American Journal of Emergency Medicine, 37(2), 321–326. https://doi.org/10.1016/j.ajem.2018.11.004
- Sagristà-Sauleda, J., Mercé, J., Permanyer-Miralda, G., & Soler-Soler, J. (2000). Clinical clues to the causes of large pericardial effusions. The American journal of medicine, 109(2), 95–101. https://doi.org/10.1016/s0002-9343(00)00459-9
- Lazaros, G., Vlachopoulos, C., Lazarou, E., & Tsioufis, K. (2021). New Approaches to Management of Pericardial Effusions. Current cardiology reports, 23(8), 106. https://doi.org/10.1007/s11886-021-01539.
- Adler, Y., et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). European heart journal, 36(42), 2921–2964. https://doi.org/10.1093/eurheartj/ehv318
- Stolz, L., Valenzuela, J., Situ-LaCasse, E., Stolz, U., Hawbaker, N., Thompson, M., & Adhikari, S. (2017). Clinical and historical features of emergency department patients with pericardial effusions. World journal of emergency medicine, 8(1), 29–33. https://doi.org/10.5847/wjem.j.1920-8642.2017.01.005
- Roy, C. L., Minor, M. A., Brookhart, M. A., & Choudhry, N. K. (2007). Does this patient with a pericardial effusion have cardiac tamponade?. JAMA, 297(16), 1810–1818. https://doi.org/10.1001/jama.297.16.1810
- McGee, S. R. (2021). Evidence-based Physical Diagnosis. United States: Elsevier.
- 1.Armstrong, W. F., Schilt, B. F., Helper, D. J., Dillon, J. C., & Feigenbaum, H. (1982). Diastolic collapse of the right ventricle with cardiac tamponade: an echocardiographic study. Circulation, 65(7), 1491–1496. https://doi.org/10.1161/01.cir.65.7.14912.
- Singh, S., Wann, L. S., Schuchard, G. H., Klopfenstein, H. S., Leimgruber, P. P., Keelan, M. H., Jr, & Brooks, H. L. (1984). Right ventricular and right atrial collapse in patients with cardiac tamponade–a combined echocardiographic and hemodynamic study. Circulation, 70(6), 966–971. https://doi.org/10.1161/01.cir.70.6.966.
- P.J. Engel, et al.Echocardiographic study of right ventricular wall motion in cardiac tamponade. Am J Cardiol, 50 (5) (1982), pp. 1018-1021.
- Mercé, J., Sagristà-Sauleda, J., Permanyer-Miralda, G., Evangelista, A., & Soler-Soler, J. (1999). Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: implications for the diagnosis of cardiac tamponade. American heart journal, 138(4 Pt 1), 759–764. https://doi.org/10.1016/s0002-8703(99)70193-6
- 5.Himelman, R. B., Kircher, B., Rockey, D. C., & Schiller, N. B. (1988). Inferior vena cava plethora with blunted respiratory response: a sensitive echocardiographic sign of cardiac tamponade. Journal of the American College of Cardiology, 12(6), 1470–1477. https://doi.org/10.1016/s0735-1097(88)80011-1
- Vakamudi, S., Ho, N., & Cremer, P. C. (2017). Pericardial Effusions: Causes, Diagnosis, and Management. Progress in cardiovascular diseases, 59(4), 380–388. https://doi.org/10.1016/j.pcad.2016.12.009